Cell capacity is of limited use if a battery pack cannot deliver the stored energy effectively; a battery also needs low internal resistance. Measured in milliohms (mΩ), resistance is extremely important the higher the C rate of the battery; the lower the resistance, the less restriction the pack encounters. This is especially important in heavy loads such as power tools and electric powertrains. High resistance causes the battery to heat up and the voltage to drop under load, this is bad for the cell, and the battery, this is what causes degradation and aging, loss of performance, and ultimately EOL(end of life)
A grade (what we now call Automotive Grade) LiFePo4 has a very low internal resistance and the battery responds well to high-current bursts that last for a few seconds to a few minutes (see the individual cell specification sheet). Compared to LFP Lead acid and inherent sluggishness, however, lead acid does not perform well on a sustained high current discharge; the battery soon gets tired and needs rest to recover. LFP however, suffers much less, And A-grade LFP is sorted by the factory because it meets the manufacturer’s specifications. This tells the manufacturer a lot about the cell, its expected performance, and its lifespan.
LFP is highly efficient and can have different performance characteristics
If we look at the A-grade EVE LF280 cells we can see the performance and efficiency. Very high!!!
Discharge capacity/nominal
capacity×100%
A)0.33CA ≥100%
B)0.5CA ≥98%
C)1CA ≥97%
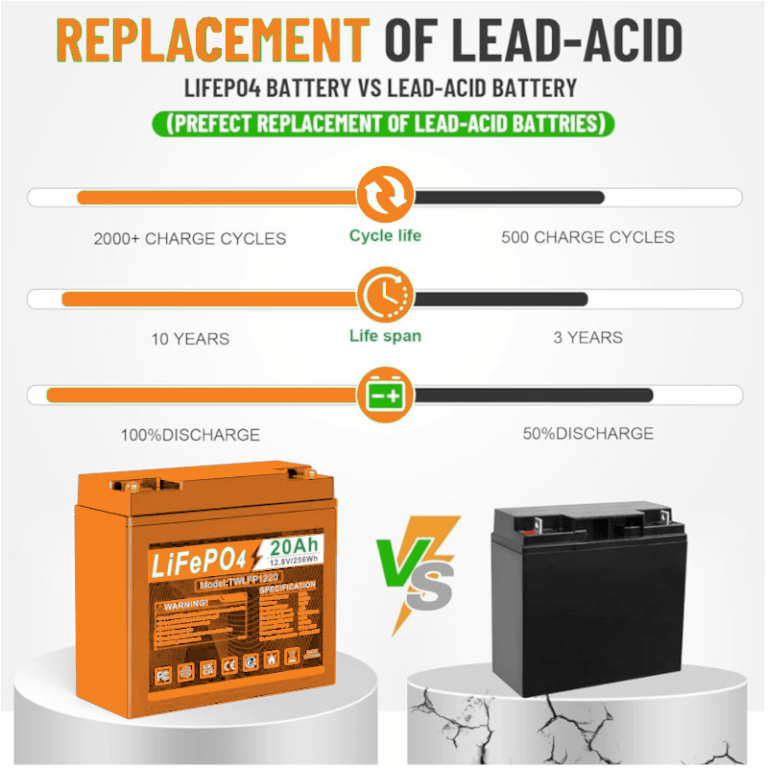
We need to compare Lead Acid again for learning purposes, Some sluggishness is apparent in all batteries at different degrees but it is especially pronounced with lead acid. This hints that power delivery is not based on internal resistance alone but also on the responsiveness of the chemistry, as well as temperature. In this respect, nickel- and lithium-based technologies are more responsive than lead acid.
The internal resistance of Lithium-based batteries also increases with use and aging but improvements have been made with electrolyte additives to keep the buildup of films on the electrodes under control. With all batteries, SoC affects the internal resistance. Lithium has higher resistance at full charge and also at end of discharge with a low resistance area in the middle. This is important to note, as when you are caring for the cells, you can very simply make the judgment that keeping your Lithium cells inside the 80% window is going to minimise degradation.
The 10%-80%-10% rule for Lithium is a good one to follow. This means try to keep you cells between 10% and 90% State of Charge.